Biosimilars, Patient Choice, And Health Care Sustainability
Education and Advocacy Increasing education around biosimilars, a new category of drug products.
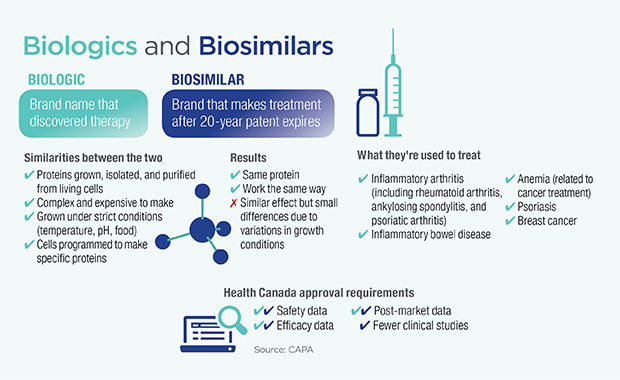
Biologics, complex drugs derived from biological sources, have been providing medical benefit for over a century. In the 1980s and 1990s, however, the field exploded as laboratory genetic recombination technology greatly expanded the tools available to researchers. And today, with the patents on these drugs beginning to expire, the floodgates have opened for competitors to enter the market with biosimilars, their own versions of these often expensive biologics. The biosimilars, however, raise some unique issues for doctors, patients, and regulators.
“Biosimilars are developed with the intention of being as close as possible to the reference biologic, but due to their biologic origin, they will never be an exact copy,” says Catherine Parker, Director General of the Biologics and Genetic Therapies Directorate at Health Canada. “There are challenges in regulating something that is extremely similar, but not identical, to another approved drug.”
Same role, different drug
The regulation process requires a biosimilar both to have a similar chemical structure to the reference biologic and to show the same safety and efficacy in clinical trials. The idea is to ensure that the chemical differences that arise from the manufacturing process do not affect patient outcomes. Still, unlike generics for small molecule drugs, they can never be perfectly identical.
“We are pretty firm in our classification of these biosimilars that we do not consider them to be generics,” says Parker. “At the same time, it makes a lot of sense to think of biosimilars as filling the same role that generics do. There is an opportunity for biosimilars to provide more affordable versions of expensive drugs. And the availability of biosimilars is a precaution against drug shortages, especially considering that many biologics are expensive and difficult to produce on a scale to satisfy the global market.”
Switching treatments should be a choice
For patients, doctors, and pharmacists, this distinction between biosimilars and generics is an important one. Everyone is eager to have more options, and less expensive ones, for treatment. Those who have been successfully treating their conditions with biologics are not, however, eager to be pressured into switching to a biosimilar for cost savings alone. Rheumatoid arthritis, an inflammatory disease that affects roughly one in ten Canadians, has become a sort of test bed for thinking about this issue.
Three of the first five biosimilars approved for use in Canada are used for the treatment of rheumatoid arthritis, a disease where differing reactions to similar medications are already common. The question of interchangeability, specifically whether a prescription for a biologic can be filled with a biosimilar, is thus a hot one. “In our disease, switching medications has never been a positive thing,” says Linda Wilhelm, President of the Canadian Arthritis Patient Alliance. “To switch any drug for non-medical reasons is an ongoing concern for patients. For biologic-naive patients, however, biosimilars present a great option for cost savings.”
Indeed, patients who are starting on a biologic for the first time have the most to gain from biosimilars. The stringent approval process ensures that the biosimilar is of equal effectiveness overall, and any individual patient could just as easily react better to the biosimilar as vice versa. “If someone has rheumatoid arthritis and will start on a biologic, often initiating the biosimilar is a good way to get them a product that may be very helpful for them while also saving money,” says Dr. Janet Pope, Chief of the Division of Rheumatology at St. Joseph’s Health Care in London, ON.
While a biosimilar is indeed a different drug from the reference biologic, it technically fills the exact same medical role as the biologic, with the same effectiveness and at a lower cost. Having these new drugs available on the market benefits everyone, so long as the question of interchangeability is treated with the seriousness it deserves.
